Speed-Dating for AI Solutions in Healthcare: A New Podcast Series from UroAI

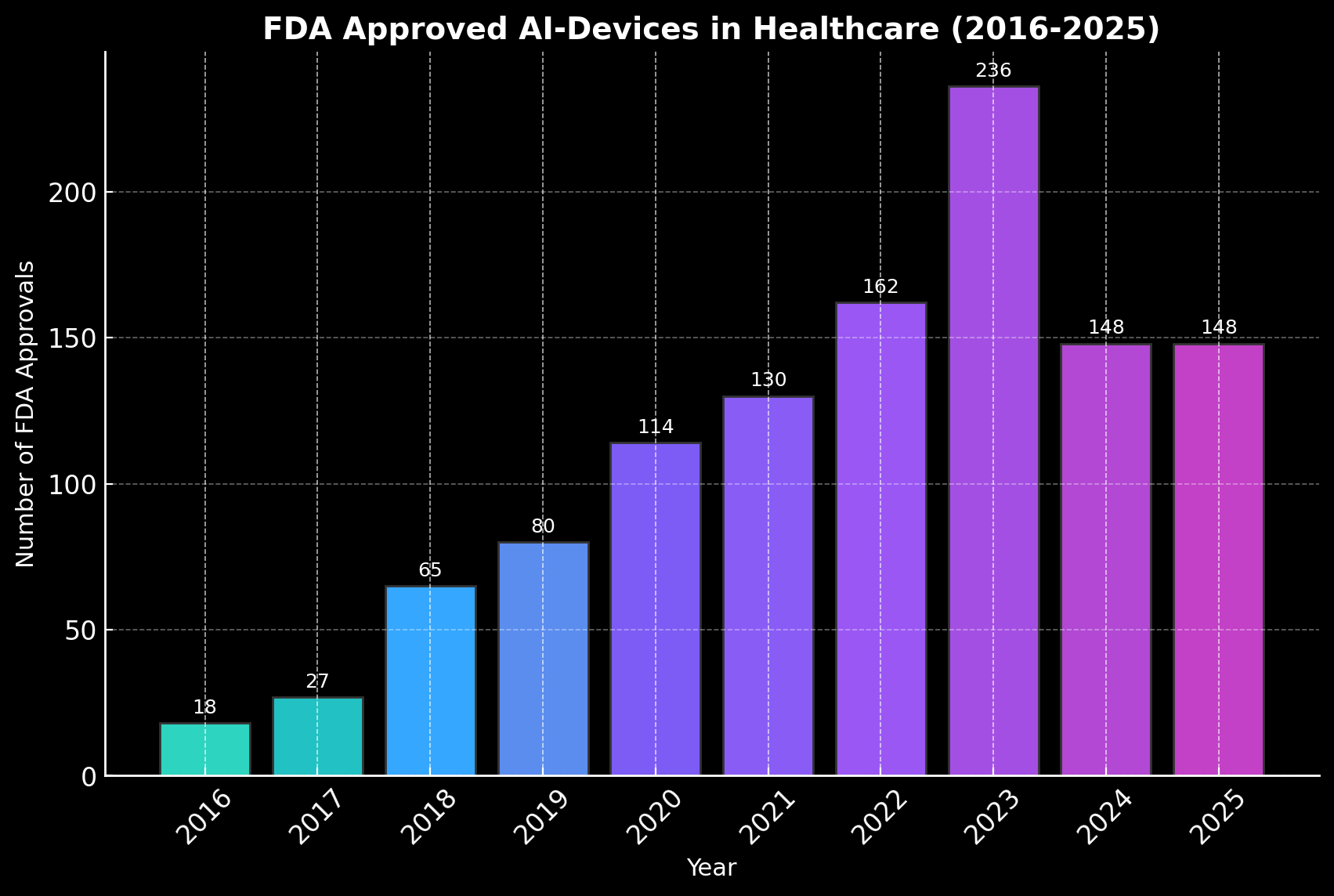
The number of FDA-cleared AI medical devices has grown rapidly in recent years. In 2022, 162 solutions were approved, followed by a record 236 in 2023. Even in 2024 and 2025, nearly 150 devices were authorized each year—levels far above the modest counts of the pre-2020 era.
This surge signals that artificial intelligence is no longer experimental in healthcare. It is entering clinical practice at scale. But with so many new products arriving, the pressing question becomes: which of these solutions can truly demonstrate evidence, safety, and usability?
To bring clarity, UroAI is launching AI Speed-Dating for Healthcare—a series of concise, 15-minute conversations with AI companies. Each discussion follows a consistent structure, grounded in The Magnificent Five Questions:
1- What healthcare problem are you solving, and how do you measure success?
2- What evidence supports your claims—peer-reviewed studies, clinical trials, or real-world data?
3- How do you handle data privacy, security, and regulatory compliance (HIPAA, GDPR, FDA, etc.)?
4- How does your AI integrate into the daily workflow of healthcare professionals?
5- What are the limitations of your system, and how do you avoid bias or unintended harm?
By asking every company these same questions, the series makes it possible to compare approaches and cut through the noise. Each interview is designed to move quickly while staying substantive, revealing not just what AI companies hope to achieve but how prepared they are for real-world deployment.
The first episodes of AI Speed-Dating for Healthcare will be available soon at UroAI.org.